Active Projects
BCL-xL-Regulated Apoptosis in Cerebellar Development and Medulloblastoma Treatment
2018/06/01-2023/04/30 R01NS102627 National Institute of Neurological Disorders and Stroke (NINDS)
GERSHON, TIMOTHY (PI), SOKOLSKY-PAPKOV, MARINA (CPI)
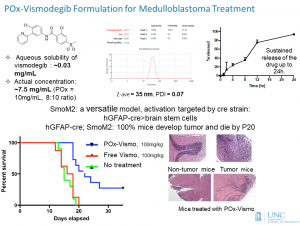 This grant will investigate the regulation of apoptosis during cerebellar development and in medulloblastoma, in order to gain new information on the pathogenesis of microcephaly and on brain tumor treatment. Medulloblastoma, the most common malignant brain tumor in children, arises from cerebellar progenitors that proliferate in the postnatal brain. We propose that cerebellar progenitors and medulloblastoma cells share a specialized mechanism of apoptosis regulation that makes the developing brain susceptible to growth failure and also makes medulloblastoma vulnerable to radiation and chemotherapy. Directly targeting this apoptosis mechanism may be a new way to treat medulloblastoma with greater efficacy and reduced toxicity. We have shown that neural progenitors and medulloblastoma cells maintain a “primed-for- death” state, in which the pro-apoptotic protein BAX is constitutively activated. These cells depend on anti-apoptotic proteins to prevent BAX from inducing spontaneous apoptosis. In our preliminary studies, we deleted the anti-apoptotic protein Bcl-xL in cerebellar progenitors to determine if BCL-xL is required for cerebellar development, and if targeting BCL-xL can impair medulloblastoma growth. We found that Bcl-xL deletion caused cerebellar progenitors to die as they exited the cell cycle. This effect blocked cerebellar growth, but surprisingly did not fully prevent medulloblastomas from growing in medulloblastoma-prone mice. Also surprising was that Bcl-xL-deleted progenitors showed increased proliferation. Based on these findings, in Aim 1 we propose to identify additional apoptosis regulators that work with BCL-xL to govern the survival of cerebellar progenitors and medulloblastoma cells. In Aim 2, we will test the hypothesis that Bcl-xL-deleted progenitors have increased proliferation because BCL-xL is required for the process of differentiation. BCL-xL has been implicated in mitochondrial function, and we have previously shown that oxidative metabolism plays an essential role in the differentiation of cerebellar progenitors. We will block apoptosis by Caspase inhibition and then determine whether BCL-xL is required for the transition from aerobic glycolysis to oxidative phosphorylation during progenitor differentiation. In Aim 3, we will use a primary mouse tumor model to examine whether inducing differentiation in medulloblastoma increases the anti-tumor effect of Bcl-xL deletion. We will also test a brain-permeant, nanoparticle-delivered BCL-xL inhibitor that we have developed as a potential medulloblastoma therapy. These Aims will show how BCL-xL regulates progenitor survival during brain growth, and test the hypothesis that BCL-xL can be targeted to improve medulloblastoma therapy.
This grant will investigate the regulation of apoptosis during cerebellar development and in medulloblastoma, in order to gain new information on the pathogenesis of microcephaly and on brain tumor treatment. Medulloblastoma, the most common malignant brain tumor in children, arises from cerebellar progenitors that proliferate in the postnatal brain. We propose that cerebellar progenitors and medulloblastoma cells share a specialized mechanism of apoptosis regulation that makes the developing brain susceptible to growth failure and also makes medulloblastoma vulnerable to radiation and chemotherapy. Directly targeting this apoptosis mechanism may be a new way to treat medulloblastoma with greater efficacy and reduced toxicity. We have shown that neural progenitors and medulloblastoma cells maintain a “primed-for- death” state, in which the pro-apoptotic protein BAX is constitutively activated. These cells depend on anti-apoptotic proteins to prevent BAX from inducing spontaneous apoptosis. In our preliminary studies, we deleted the anti-apoptotic protein Bcl-xL in cerebellar progenitors to determine if BCL-xL is required for cerebellar development, and if targeting BCL-xL can impair medulloblastoma growth. We found that Bcl-xL deletion caused cerebellar progenitors to die as they exited the cell cycle. This effect blocked cerebellar growth, but surprisingly did not fully prevent medulloblastomas from growing in medulloblastoma-prone mice. Also surprising was that Bcl-xL-deleted progenitors showed increased proliferation. Based on these findings, in Aim 1 we propose to identify additional apoptosis regulators that work with BCL-xL to govern the survival of cerebellar progenitors and medulloblastoma cells. In Aim 2, we will test the hypothesis that Bcl-xL-deleted progenitors have increased proliferation because BCL-xL is required for the process of differentiation. BCL-xL has been implicated in mitochondrial function, and we have previously shown that oxidative metabolism plays an essential role in the differentiation of cerebellar progenitors. We will block apoptosis by Caspase inhibition and then determine whether BCL-xL is required for the transition from aerobic glycolysis to oxidative phosphorylation during progenitor differentiation. In Aim 3, we will use a primary mouse tumor model to examine whether inducing differentiation in medulloblastoma increases the anti-tumor effect of Bcl-xL deletion. We will also test a brain-permeant, nanoparticle-delivered BCL-xL inhibitor that we have developed as a potential medulloblastoma therapy. These Aims will show how BCL-xL regulates progenitor survival during brain growth, and test the hypothesis that BCL-xL can be targeted to improve medulloblastoma therapy.
Carolina Center for Cancer Nanotechnology Excellence: Nano Approaches to Modulate Host Cell Response for Cancer Therapy. Project 4: High Capacity Polymeric Micelle Therapeutics for Lung Cancer
2015/09/15-2020/07/31 U54CA198999 National Cancer Institute (NCI)
HUANG, LEAF (PI), TEPPER, JOEL (CPI), KABANOV, ALEXANDER V (PI, Project 4), WANG, ANDREW (CPI), PECOT, CHAD (CPI)
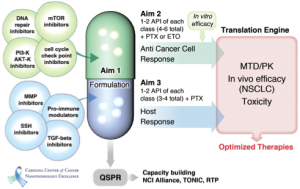 The central goal of this project is to improve systemic therapies for non-small cell lung cancer (NSCLC) using combinations of potent anticancer agents, chemosensitizers, and agents that target the tumor microenvironment (TME). Many highly promising small-molecule cancer-targeting therapeutics fail due to poor solubility, stability and other delivery related problems. Recent advances in nanoparticle (NP) drug delivery vehicles provide a unique opportunity to “rescue” these agents for clinical application. This is the focal point of our research, which is to use NPs that can incorporate such agents and preferentially deliver them to tumors. Our group has successfully developed a novel drug delivery platform based on poly(2-oxazoline) (POx) polymeric micelles, that is well suited for the delivery of poorly soluble active drugs, such as paxclitaxel (PTX). Compared to conventional formulations and other NP platforms, our POx platform is unique in its high drug loading capacity. Our preliminary data showed that such high drug loading translated into lower toxicity and high therapeutic efficacy when we compared POx/PTX to both Taxol® and Abraxane®. The National Characterization Laboratory has evaluated the PTX formulation using our lead POx block copolymer of poly(2-butyl-2-oxozaline) (BuOx) and poly(2-methyl-2-oxazoline) (MeOx), and concluded that both the copolymer and formulation lack immunological and hematological toxicities. Significantly, we have shown that multiple agents can be co-formulated within the same POx micelles, enabling co-delivery of anticancer agents, chemosensitizers, and TME-modifying compounds. Such combinations can increase cancer cell cytotoxicity and modify the TME to enhance tumor control. We hypothesize that POx micelles can serve as a powerful and versatile platform for delivery of such agents. We assembled a cross-disciplinary team of physician-scientists and experts in nanotechnology, pharmacology, and chemoinformatics, and will focus our research efforts on using POx micelle therapeutics in NSCLC, a disease that despite treatment advances, still has a very poor outcome. The Specific Aims are: 1. Develop a predictive computational model for rapid selection and incorporation of new and existing anticancer agents, chemosensitizers, and TME modifiers into POx micelles. 2. Evaluate chemosensitizers and anticancer agents incorporated in POx micelles as therapeutic modalities to improve treatment of NSCLC. 3. Evaluate polymeric micelle formulations of agents that target the TME as a treatment strategy. The computational model for rational design of formulations will drastically increase the throughput and allow us to further develop new anti-cancer therapeutics through trans-Alliance collaborations and other NCI mechanisms. Successful therapeutics will be advanced for further development.
The central goal of this project is to improve systemic therapies for non-small cell lung cancer (NSCLC) using combinations of potent anticancer agents, chemosensitizers, and agents that target the tumor microenvironment (TME). Many highly promising small-molecule cancer-targeting therapeutics fail due to poor solubility, stability and other delivery related problems. Recent advances in nanoparticle (NP) drug delivery vehicles provide a unique opportunity to “rescue” these agents for clinical application. This is the focal point of our research, which is to use NPs that can incorporate such agents and preferentially deliver them to tumors. Our group has successfully developed a novel drug delivery platform based on poly(2-oxazoline) (POx) polymeric micelles, that is well suited for the delivery of poorly soluble active drugs, such as paxclitaxel (PTX). Compared to conventional formulations and other NP platforms, our POx platform is unique in its high drug loading capacity. Our preliminary data showed that such high drug loading translated into lower toxicity and high therapeutic efficacy when we compared POx/PTX to both Taxol® and Abraxane®. The National Characterization Laboratory has evaluated the PTX formulation using our lead POx block copolymer of poly(2-butyl-2-oxozaline) (BuOx) and poly(2-methyl-2-oxazoline) (MeOx), and concluded that both the copolymer and formulation lack immunological and hematological toxicities. Significantly, we have shown that multiple agents can be co-formulated within the same POx micelles, enabling co-delivery of anticancer agents, chemosensitizers, and TME-modifying compounds. Such combinations can increase cancer cell cytotoxicity and modify the TME to enhance tumor control. We hypothesize that POx micelles can serve as a powerful and versatile platform for delivery of such agents. We assembled a cross-disciplinary team of physician-scientists and experts in nanotechnology, pharmacology, and chemoinformatics, and will focus our research efforts on using POx micelle therapeutics in NSCLC, a disease that despite treatment advances, still has a very poor outcome. The Specific Aims are: 1. Develop a predictive computational model for rapid selection and incorporation of new and existing anticancer agents, chemosensitizers, and TME modifiers into POx micelles. 2. Evaluate chemosensitizers and anticancer agents incorporated in POx micelles as therapeutic modalities to improve treatment of NSCLC. 3. Evaluate polymeric micelle formulations of agents that target the TME as a treatment strategy. The computational model for rational design of formulations will drastically increase the throughput and allow us to further develop new anti-cancer therapeutics through trans-Alliance collaborations and other NCI mechanisms. Successful therapeutics will be advanced for further development.
Targeting Tumor Associated Fibroblasts to Enhance Therapy
2019/06/01-2021/05/31 Eshelman Institute for Innovation
HUANG, LEAF (CPI), KABANOV, ALEXANDER (CPI), KIM, WILLIAM (CPI)
Tumor associated fibroblasts (TAFs) play a central role in drug resistance in bladder cancer. Dr. William Kim will study the essential role of TAFs in the resistance of bladder cancer to immunotherapy. Dr. Alexander Kabanov will use polymer micelles to deliver immunostimulatory drugs to enhance the immunotherapy for bladder cancer. Dr. Leaf Huang will use traditional Chinese medicines to induce immunogenic cell death of the bladder cancer cells to enhance immunotherapy for bladder cancer.
Targeted Core Shell Nanogels for Triple Negative Breast Cancer
2015/08/14-2020/07/31 U01CA198910 National Cancer Institute (NCI)
KABANOV, ALEXANDER V (PI), BRONICH, TATIANA (CPI), LIU, RIHE (CPI)
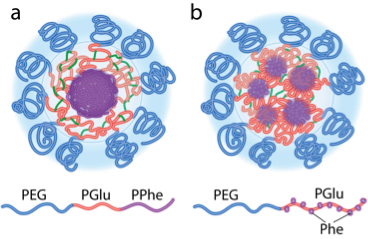
The central goal of this project is to improve systemic therapies of cancer using soft nanomaterials that can deeply penetrate into tumors and deliver potent anticancer agents to targeted cancer cells. Many small-molecule therapeutics that were highly promising for cancer therapy, eventually failed clinical translation due to toxicity, poor solubility, stability and other delivery related problems. Recent advances in nanoparticle drug carriers provide a unique opportunity to “rescue” these agents and enable their clinical application. This is the focal point of our research using nanocarriers that can incorporate such agents, and preferentially deliver them to tumors. Our group has successfully developed a novel platform for drug delivery that uses aqueous polymeric gel nanoparticles, core-shell nanogels (CSNGs). CSNGs are manufactured through a proprietary self-assembly process and can be readily filled with various drug payloads. They are water-swollen and are practically non-adhesive, which may diminish their off-target side effects. We hypothesize that (a) the systemic and tumor flow dynamics of CSNGs will be a function of their molecular architecture and mechanical properties (b) and that these properties can be rationally controlled to modify the PK, distribution and tumor penetration of the CSNGs and the drugs they deliver. We will focus our research efforts on using CSNGs in the triple negative breast cancer (TNBC), a disease that despite treatment advances, still has a very poor outcome. As targeting strategies we will use the novel single-domain polypeptide antagonists of the EGFR and HER3 that are frequently overexpressed in TNBC and are associated with higher risk of mortality in TNBC. We assembled a cross-disciplinary team of physician-scientists and experts in nanotechnology and pharmacology, and will focus our research efforts on using CSNGs-based therapeutics in TNBC, a disease that despite treatment advances has a very poor outcome. The specific aims are: 1. Determine how the molecular architecture and mechanical properties of polypeptide-based CSNGs affects their ability to load, deliver and release therapeutic cargos. 2. Determine how the structure and mechanical properties of the drug-loaded CSNGs affect the in vivo PK and tumor distribution of the drugs and nanogels in murine models of TNBC. 3. Develop EGFR and HER3 targeted drug-loaded CSNGs with maximal tumor penetration, maximal delivery of drug payload to tumors and potent anti-tumor activity in TNBC. These integrative efforts will address major barriers in developing novel nanotechnology platforms for the treatment of TNBC, and facilitate our understanding of the cancer biology and the mechanisms of in vivo delivery. If successful, we will determine a new targeted formulation of chemotherapeutic drugs in CSNGs that may be highly effective in treatment of TNBC. The results will then be reviewed with our clinical advisors for further consideration of these formulations for translational and clinical development.
Carolina Cancer Nanotechnology Training Program (C-CNTP)
2015/07/01-2025/06/30 T32CA196589 National Cancer Institute (NCI)
KABANOV, ALEXANDER V (PI), WANG, ANDREW (CPI)
This proposal seeks competing continuation of the NIH T32 Carolina Cancer Nanotechnology Training Program (C-CNTP). The program started in 2015 with the support of the NIH Ruth L. Kirschstein National Research Service Award. The goal of the program is to make a major contribution to the growth of the cancer nanotechnology workforce by providing training and research experience to a highly select cohort of postdoctoral fellows. During four years since its initiation the program has recruited 15 excellent fellows of whom 5 have completed training and moved into tenure track assistant professors, industry and FDA positions, 8 continue training with the NIH T32 support and 2 are in the final stages of training with other support sources in the labs of their mentors. We have assembled a team of 20 outstanding Program faculty from 11 departments and 3 schools at the University of North Carolina Chapel Hill with specific expertise in physical and material sciences, chemistry, pharmacoengineering, drug delivery, computational modeling as well as basic biomedical research and clinical science, all of whom have demonstrated strong interests, capabilities and collaborations at the interface between nanoscience and cancer. The objectives of the C-CNTP are to: 1) recruit an elite group of talented postdoctoral fellows from diverse backgrounds with PhD, MD or PharmD and provide them with outstanding postdoctoral experience including focused didactic training and co-mentored research experience with faculty mentors from complementary fields; 2) provide each trainee with intensive introductory training with online video library of recorded lectures followed by workshops and didactic courses to remediate differences in their backgrounds and to deepen the knowledge and understanding in the key areas of cancer nanotechnology; and 3) facilitate transition of trainees to independence by providing them with opportunities to a) conduct original cancer nanotechnology research projects; b) apply for the individual cancer nanotechnology Pilot Grants within C-CNTP, and c) acquire written and oral communications skills needed to publish manuscripts, report results, and write successful individual extramural support applications focused on problems of cancer nanotechnology. We are committed to developing a world-class postdoctoral training program that capitalizes on the existing strengths in cancer nanotechnology research, consolidates diverse research and education resources across several academic units, and becomes a significant contributor to addressing the Nation’s research needs in cancer nanotechnology.
Liposomal Doxorubicin and Pluronic Combination for Cancer Therapy
2015/01/01-2020/12/31 R01CA184088 National Cancer Institute (NCI)
KABANOV, ALEXANDER V (PI), ZAMBONI, WILLIAM (CPI)
PEGylated liposomal doxorubicin (Doxil, PLD) is used clinically to treat ovarian cancer (OC) and breast cancer (BC); however, the response rates of PLD treatment in both diseases need to be improved. Based on strong preliminary data, we propose a novel simple strategy to increase the efficacy of PLD treatment by promoting the release of the active ingredient (doxorubicin, (Dox)) from the liposomal particles directly within the tumor matrix, while concurrently sensitizing this tumor to the drug. In this strategy, amphiphilic Pluronic block copolymers (poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide), PEO-PPO-PEO) are intravenously (IV) administered after the PLD treatment, when the concentration of the liposomal drug within the tumor reaches its maximum. We posit, the copolymer then depots into tumors, incorporates into the PLD particles, and promotes the encapsulated drug release, thus increasing drug bioavailability and improving the tumor response. Moreover, a combination of Dox and Pluronic generated within tumors is highly potent in eliminating multidrug resistant (MDR) and tumor-initiating cells (TIC) that can further improve the therapeutic outcomes in cancer. Our objectives are: to determine mechanism by which administration of Pluronic after the PLD increases the anti-tumor activity; provide proof of principle using genetically engineered mouse models (GEMMs) of OC and BC that closely represent biology and microenvironment of solid tumors in patients; and select Pluronic compositions and treatment regimen to maximize the translational and clinical outcomes. The aims will: 1) determine in vitro release kinetics of Dox from PLD and in vivo pharmacokinetics (PK) of Pluronic to select the best Pluroinic composition, doses and schedule; 2) evaluate PK of PLD alone and in combination with the selected Pluronic(s) to determine the amount of Dox released from liposomes in plasma and drug exposure in tumor; 3) evaluate the anti-tumor activity and safety of the proposed treatments; and 4) determine whether administration of Pluronic after PLD results in depletion of TIC, and decreases tumorigenicity and aggressiveness of cancer cells. The proposed combination therapy if successful has high potential for translation to clinical studies, since it is simple, can improve efficacy of clinically available PLD (such as Doxil), and is likely to be safe, since Pluronics were shown to be safe in clinical trials of non-liposomal Dox/Pluronic formulation, SP1049C.
Targeted Magneto-Mechanic Nanotherapeutics for Cancer
2017/08/01-2020/07/31 R21CA220148 National Cancer Institute (NCI)
KABANOV, ALEXANDER V (PI)
We propose a new nanomedicine paradigm that non-heating super low frequency alternating magnetic field (AMF) applied to superparamagnetic nanoparticles (MNPs) can lead to mechanical forces and carry out mechanical work at the nanoscale resulting in remotely actuated changes of structure and function of surrounding biological macromolecules and supramolecular structures. In prior work we discovered a new mechanism of toxicity of MNPs in AMF to cancerous cells that involves cytoskeletal disruption and subsequent cell death and can be enacted upon cancerous cells while leaving healthy cells intact. We use this approach to kill cancer cells that are mechanically softer than their benign counterparts and more sensitive to mechano- transduction leading to cytoskeletal damage and cell death. Notably, our MNP system responds to super low frequency and low amplitude magnetic fields with relatively short exposure times, which can greatly diminish possible side effects such as non-specific heating of surrounding tissues. The effect was observed with small magnetite MNPs of 7 to 8 nm in diameter that can be conjugated with targeting antibodies to tumor antigens and delivered systemically to the tumors. This exploratory project aims to obtain the proof of concept for remotely actuated magneto-mechanical cancer nanotherapeutics and use of MNPs for magneto-mechanical destruction of tumors in vivo. The aims are designed to 1) determine antitumor effects of MNPs induced by super low frequency AMF in an animal model of breast cancer; 2) employ multimodal magnetic field capability accessing alternating current (AC) and direct current (DC) magnetic fields and their combination treatments to increase the treatment outcomes; and 3) develop targeted polymer-coated, biocompatible magnetite MNPs for efficient systemic delivery into HER2 positive tumors and their magneto-mechanical treatment to inhibit tumor growth. The proposal builds upon the existing collaboration between the investigators at M.V. Lomonosov Moscow State University (MSU) and University of North Carolina-Chapel Hill (UNC) where both teams converge their synergistic expertise in chemistry and physics of superpamagnetic nanomaterials, engineering of uniform magnetic field space, polymer therapeutics, drug delivery and cancer nanotechnology to demonstrate feasibility of this new technology for cancer therapy.
Nanoparticle Delivery of Cas9 and Therapeutic gRNAs to the Brain
2018/06/01-2020/05/31 Eshelman Institute for Innovation
KABANOV, ALEXANDER V (PI)
We are developing a therapy for Angelman syndrome, a neurodevelopmental disorder occurring due to deletion of maternal Ube3a gene. We propose to unsilence the paternal gene that is functional but epigenetically silenced. Working towards this, we are exploring a novel technology to deliver gene-editing machinery to neurons in the brain. If successful, our strategy could provide the first ever treatment, and possibly cure, for Angelman syndrome and can also be applied to autism and other neurodevelopmental disorders that currently have no cure.
Targeting of Inflamed Monocytes for Gene Therapy of Cancer
2019/06/01-2020/05/31 Eshelman Institute for Innovation
KABANOV, ALEXANDER V (PI)
In this proposal we aim to overcome the major drawbacks to gene therapy to tumors by developing a systemic gene delivery technology that can actively target circulating monocytes that carry disease-induced inflammation markers. The mRNA or DNA will be incorporated into inert polyplexes and decorated with ligands expressed on blood-born inflamed monocytes. We posit that the systemically manipulated monocytes will extravasate into tumors, differentiate into tumor-associated macrophages and horizontally transfer the gene of interest to the acceptor cells (such as cancer cells, fibroblasts and immune cells) resulting in the gene expressed in the tumor tissue.
